DiaPlexC™ Avellino Corneal Dystrophy (ACD) Genotyping Kit
Multiplex allele-specific PCR based assay system for the genotyping of the ACD gene SNP related to avellino corneal dystrophy
CE-IVD
Specification
| DiaPlexC™ Avellino Corneal Dystrophy (ACD) PCR Genotyping Kit | ||
|---|---|---|
| Detection target | SNP of R124 (βigh3 coding gene codon 124, exon4) | |
| Registration | CE-IVD | |
| Detection technology | Conventional (End-point) Multiplex PCR | |
| Specimen type | Blood, Buccal epithelial cell, Hair (root) |
|
| Compatible instruments* | ABI Veriti thermal Cycler (Applied Biosystems) recommended |
|
| PCR running time | ~ 1hr 30 min | |
Features
– HotStart PCR system : Ultra high specific and sensitive result
– UDG system : No carryover contamination
– Multiplex PCR : Multiple targets in a single reaction
– Reliable system : Automatic Internal control ( DiaPlex C™)
– Positive control included
– Easy-to-use master mix
– CE certification
Experimental
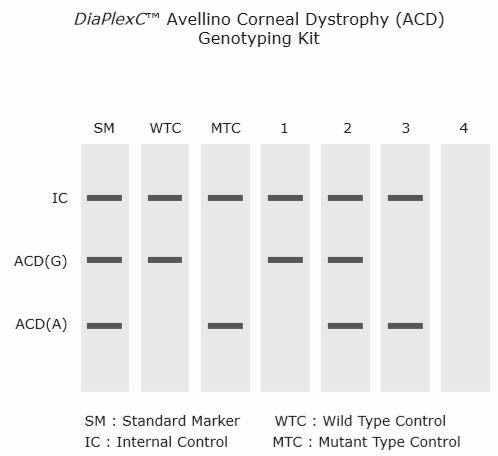
| Lane | Interpretation (detection) |
|---|---|
| 1 | ACD (G/G homozygote, normal) |
| 2 | ACD (G/A heterozygote, mutant) |
| 3 | ACD (A/A homozygote, mutant) |
| 4 | Required re-experiment |
Citation&Papers
Kocak-Atlintas AG, Kocak-Midillioglu I, Akarsu AN, Duman S.
βigh gene analysis in the different diagnosis of corneal dystrophies. Cornea 2001; 20: 64-8.
Advances in the molecular genetics of corneal dystrophies. Am J phthalmol 1999; 128: 747-54.
Konishi M, Mashima Y, Nakamura Y, et al.
Granular-lattice (Avellino) corneal dystrophy in Japanese patients. Cornea 1997; 16: 635-8.
Ordering information
| Technology | Cat. No. | Product | Contents |
|---|---|---|---|
| Conventional (End-point) PCR | SHG06-K020 (20 reaction) |
DiaPlexC™ Avellino Corneal Dystrophy (ACD) Genotyping Kit |
2X Multiplex PCR Smart mix (with UDG) (ACD) Primer Mixture (ACD) Standard Marker (ACD) Wild type Control (ACD) Mutant type Control (ACD) Nuclease free Water |
| SHG06-K100 (100 reaction) |



