DiaPlexQ™ MTC/NTM Detection Kit
Real-Time PCR based assay system for simultaneous detection of MTC and NTM complex
CE-IVD
Specification
| DiaPlexQ™ MTC/NTM Detection Kit | DiaPlexQ™ MTC/NTM Detection Kit ver 3.0 |
DiaPlexQ™ MTC/NTM Detection Kit ver 4.0 |
|
|---|---|---|---|
| Detection target | MTC (2 species): IS6110 NTM (13 species): 16S rRNA |
MTC (2 species): IS6110, MPB64 NTM (15 species): 16S rRNA |
MTC (2 species): IS6110, MPB64 NTM (6 species): 16S rRNA |
| Registration | KFDA, CE-IVD | CE-IVD | CE-IVD |
| Detection technology | Real-Time PCR |
||
| Specimen type | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage (BAL) |
||
| Analytical sensitivity | 10 copies | ||
| Compatible instruments* | ABI 7500 / 7500 Fast Real-Time PCR System (Applied Biosystems) CFX96™ Real-Time PCR System (Bio-Rad) |
||
| PCR running time | ~ 1 hr 20 min | ||
Features
– HotStart PCR system : Ultra high specific and sensitive result
– DnaFree™ system : No host genomic DNA contamination ( DiaPlexC ™)
– UDG system : No carryover contamination
– Multiplex PCR system : Multiple targets in a single reaction
– Reliable system : Automatic PCR control
– Positive control included
– DNA extraction solution included (Cat. No. SQD21-K100)
– Easy-to-use master mix
– CE certification
Experimental
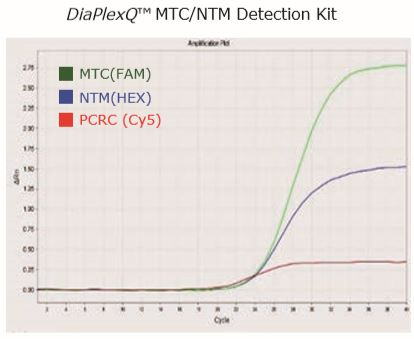
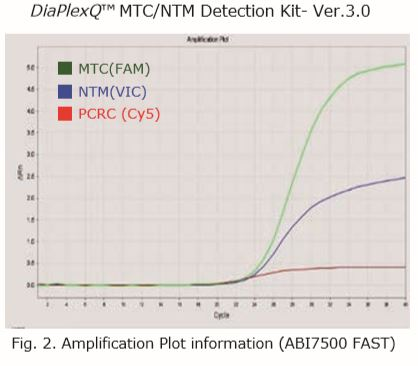
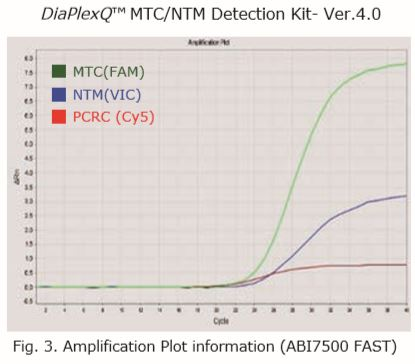
| Sample type | FAM | HEX | Cy5 | Result |
|---|---|---|---|---|
| Positive Control | + | + | + | Valid |
| Negative Control | - | - | + | Valid |
| NTC(Non-Template Control) | - | - | + | Valid |
| Sample case 1 | + | + | +/- | MTC, NTM (Co-infection) |
| Sample case 2 | + | - | +/- | MTC |
| Sample case 3 | - | + | +/- | NTM |
| Sample case 4 | - | - | + | Negative |
| Sample case 5 | - | - | - | Required re-experiment |
Citation&Papers
Korean J Clin microbial, Seong Deok Lee, Hye Young Lee, Hyun Chul Kim, Soo Young Kim,
Mycobacterium tuberculosis and Non-Tuberculous Mycobacteria by PCR Assay.
Ryan KJ, Ray CG (Editors) (2004).
Sherris Medical Microbiology (4th ed.). McGraw-Hill. Clinical Characteristics of Non-Tuberculous Mycobacterial Pulmonary Disease in a National Tuberculosis Hospital.
Sun-Pil Choi, M.D., Bong-Keun Lee M.D.1, Jin-Hong Min, M.D., Jin-Hee Kim, M.D.
Pathogenic Classification and Clinical Charateristics of Non-Tuberculous Mycobacterial Pulmonary Disease in a National Tuberculosis Hospital.
Ordering information
| Technology | Cat. No. | Product |
|---|---|---|
| Real-time PCR | SQD21-K100 | DiaPlexQ™ MTC/NTM Detection Kit (w/Ext.) |
| SQD20-K100 | DiaPlexQ™ MTC/NTM Detection Kit | |
| SQD26-K100 | DiaPlexQ™ MTC/NTM Detection Kit (w/Ext.)- Ver.3.0 | |
| SQD25-K100 | DiaPlexQ™ MTC/NTM Detection Kit- Ver.3.0 | |
| SQD28-K100 | DiaPlexQ™ MTC/NTM Detection Kit (w/Ext.)- Ver.4.0 | |
| SQD27-K100 | DiaPlexQ™ MTC/NTM Detection Kit- Ver.4.0 |



