DiaPlexC™ MTC/NTM Detection Kit
Multiplex PCR based assay system for simultaneous detection of MTC and NTM complex
Specification
| DiaPlexC™ MTC/NTM Detection Kit |
|
|---|---|
| Detection target | MTC (2 species), NTM (10 species) |
| Registration | CE-IVD |
| Detection technology | Conventional (End-point) Multiplex PCR |
| Specimen type | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage (BAL), Nasal swab, Oropharyngeal swab, Sputum |
| Analytical sensitivity | 10² - 105 copies |
| Compatible instruments* | ABI Veriti thermal Cycler (Applied Biosystems) recommended |
| PCR running time | ~ 1 hr 30 min |
Features
– HotStart PCR system : Ultra high specific and sensitive result
– DnaFree™ system : No host genomic DNA contamination ( DiaPlexC ™)
– UDG system : No carryover contamination
– Multiplex PCR system : Multiple targets in a single reaction
– Reliable system : Automatic PCR control
– Positive control included
– DNA extraction solution included (Cat. No. SQD21-K100)
– Easy-to-use master mix
– CE certification
Experimental
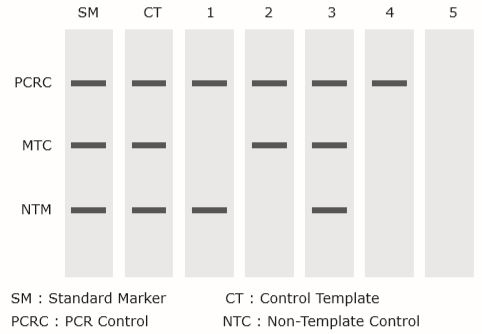
| Lane | Interpretation (detection) |
|---|---|
| 1 | NTM |
| 2 | MTC |
| 3 | MTC, NTM (Co-infection) |
| 4 | Negative (or NTC) |
| 5 | Required re-experiment |
Citation&Papers
Korean J Clin microbial, Seong Deok Lee, Hye Young Lee, Hyun Chul Kim, Soo Young Kim,
Mycobacterium tuberculosis and Non-Tuberculous Mycobacteria by PCR Assay.
Ryan KJ, Ray CG (Editors) (2004).
Sherris Medical Microbiology (4th ed.). McGraw-Hill. Clinical Characteristics of Non-Tuberculous Mycobacterial Pulmonary Disease in a National Tuberculosis Hospital.
Sun-Pil Choi, M.D., Bong-Keun Lee M.D.1, Jin-Hong Min, M.D., Jin-Hee Kim, M.D.
pathogenic Classification and Clinical Characteristics of Non-Tuberculous Mycobacterial Pulmonary Disease in National Tuberculosis Hospital
Ordering information
| Technology | Cat. No. | Product | Contents |
|---|---|---|---|
| Conventional (End-point) PCR | SMD21-K020 (20 reaction) | DiaPlexC ™ MTC/NTM Detection Kit | 2X Multiplex PCR Smart mix (with UDG) (MTC/NTM) Primer Mixture (MTC/NTM) Standard Marker (MTC/NTM) Control Template (MTC/NTM) Nuclease free Water |
| SMD21-K100 (100 reaction) |



