DiaPlexQ™ G6PD Genotyping Kit
Glucose-6-Phosphate Dehydrogenase Deficiency
G6PD deficiency is a genetic disorder caused by a lack of G6PD levels due to mutations in the X-Chromosome gene, which are associated with various clinical symptoms. The most common diseases are acute hemolytic anemia, neonatal jaundice, and hereditary nonspherocytic hemolytic anemia.
G6PD is responsible for the reduction of glutathione, an oxidative stress oxidized in red blood cells. This prevents the oxidation of hemoglobin and proteins. If G6PD is deficient, it causes irreversible damage to the red blood cell membrane and destroys red blood cells, causing abnormalities in our body.
The most serious problem of G6PD deficiency occurs when a person with G6PD deficiency is infected with malaria and does not recognize G6PD deficiency and is prescribed specific drugs (anti-malaria, sulfonamide antibiotics) for the treatment of malaria. In this case, the patient may feel symptoms such as acute hemolytic anemia due to the reaction by specific drugs other than malaria, and in severe cases, it may lead to death. G6PD deficiency is closely related to malaria-endemic areas, and for this reason, pre-diagnosis for G6PD deficiency in the area is important.
Therefore, it is important to diagnose G6PD deficiency to prevent complications. For patients with G6PD deficiency, it is best to avoid the specific drug, food, and environmental causes that cause oxidative stress in red blood cells.
G6PD deficiency is estimated to have 4 million patients globally, and 4,100 People died from G6PD Deficiency in 2013.
DiaPlexQ™ G6PD Genotyping Kit
Product Overview
SolGent Co., Ltd is developing a Fast-qPCR G6PD Genotyping Kit that can easily diagnose G6PD deficiency with POCT by adding template DNA to the supplier’s fast PCR equipment. It is technically completed, we plan to commercialize the product by registering the product certification and license after verifying the compatibility with Partner Company’s equipment.
Product Features
G6PD gene mutation can be detected per class (Class Ⅱ ∙ Ⅲ ∙ Ⅳ) in a single chip and can be checked in real time through fluorescent substance.
Test time can be reduced about 8 times compared to existing PCR equipment (existing equipment: 2 hours, Fast PCR equipment: 15 minutes)
Clinicians can easily diagnose G6PD deficiency with POCT (Weight: 3kg, Electric power can be supplied through portable battery)
Detection Target
G6PD deficiency is frequent in Africa, Southeast Asia, the Middle East, Mediterranean and the South Pacific, North and South America.
This is consistent with areas where the prevalence of malaria has recently increased.
Detection Target
G6PD deficiency is frequent in Africa, Southeast Asia, the Middle East, Mediterranean and the South Pacific, North and South America.
This is consistent with areas where the prevalence of malaria has recently increased.
Target Gene
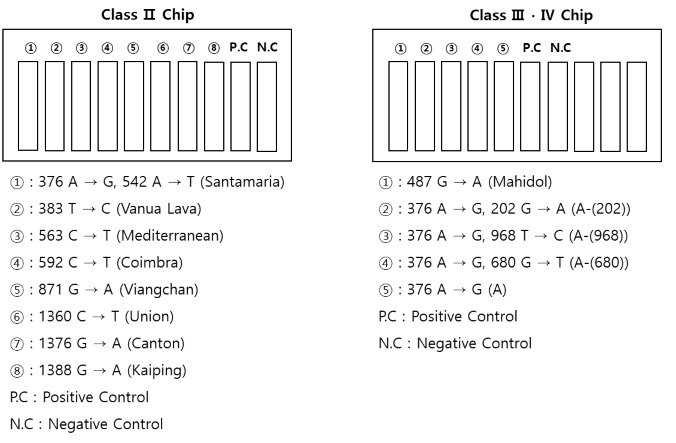
Class | Enzyme activity | Variant |
Ⅰ | Severe deficiency: with chronic nonspherocytic hemolytic anemia | Sunderland, Nara, Guadalajara |
Ⅱ | Severely deficiency: 1~10% residual enzyme activity with intermittent hemolysis | Mediterranean, Oriental variant |
Ⅲ | Moderate deficiency: 10~60% residual enzyme activity, hemolysis with stressors only | African variant |
Ⅳ | Normal activity: 60~150 enzyme activity, no clinical sequelae | A, B |
Ⅴ | Increased activity: > 150% enzyme activity, no clinical sequelae | Hektoen |
[표1] WHO classification of G6PD deficiency variant
Expected effect
DiaPlexC™ G6PD Genotyping Kit can prevent serious side effects of Anti-malarial drugs such as hemolytic anemia through a G6PD genotyping test, and you can also manage symptoms such as jaundice and pigmentation that G6PD can cause.
Result & Data Interpretation
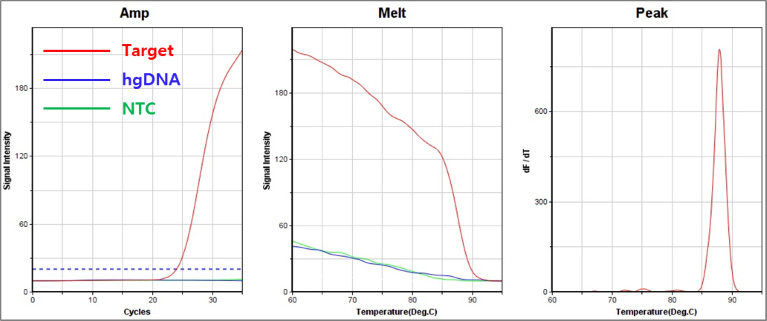
A. 592 C → T (Coimbra) : Class Ⅱ
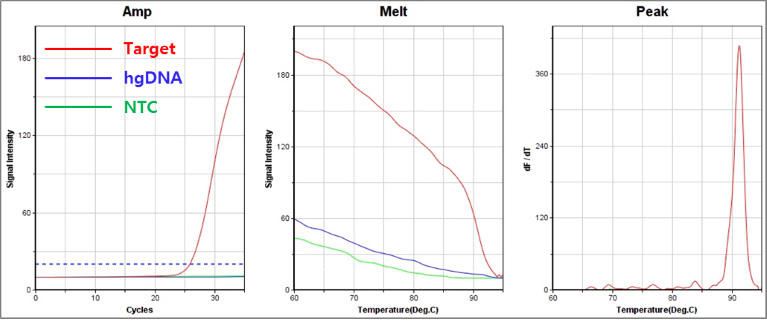
B. 376 A → G, 202 G → A : Class Ⅲ
C. 376 A → G : Class Ⅳ
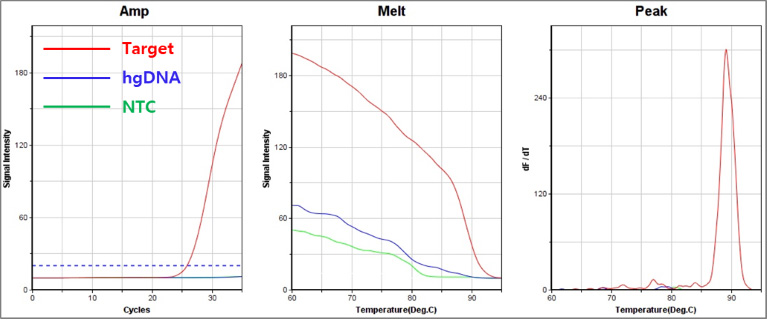
[Pic1] G6PD Detection Result using Fast-PCR Instrument of G Company
Reference
[1] Frank JE (October 2005). “Diagnosis and management of G6PD deficiency”. 《Am Fam Physician》 72 (7): 1277–82. PMID 16225031
[2]GBD 2013 Mortality and Causes of Death, Collaborators. “Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013.”. 《Lancet》 385: 117–71. PMC 4340604. PMID 25530442. doi:10.1016/S0140-6736(14)61682-2
[3] Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ 1989;67:601-11
[4] Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis 2009;42:267-78.
[5] Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008;371:64-74.
[6] Jae Min Lee MD, Glucose-6-phosphate dehydrogenase deficiency. Pediatric hematology-oncology 2015;April Number 1



